Marque(s):
- Safeheart
Fabricant:
- SAVA Vet
Diseases:
- Congestive Heart Failure In Dogs
- We deliver to any place on Earth
- Complete anonymity and data security
- Only certified products and attractive prices
- No fuss and long queues to get medicines
Safeheart® | Bitcoin Pharmacy
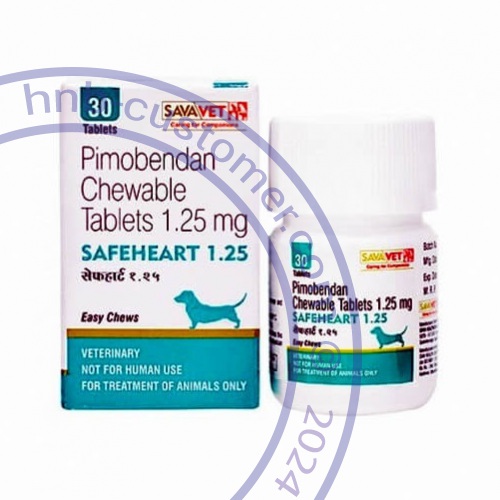
Pimobendan
| Paquet | Par comprimé | Prix | D'épargne | Achetez! |
|---|---|---|---|---|
|
30 tablet
|
€ 3.01
|
€ 90.41 € 3.01 Par comprimé
|
|
|
|
60 tablet
|
€ 2.38
|
€ 142.78 € 2.38 Par comprimé
|
€ 38.04
|
|
|
90 tablet
Free AirMail shipping
|
€ 1.90
|
€ 171.35 € 1.90 Par comprimé
|
€ 99.89
|
|
|
120 tablet
Free AirMail shipping
|
€ 1.67
|
€ 199.91 € 1.67 Par comprimé
|
€ 161.73
|


Marque(s):
- Safeheart
Fabricant:
- SAVA Vet
Diseases:
- Congestive Heart Failure In Dogs
Safeheart tablets
Safeheart (pimobendan) is indicated for the management of the signs of mild, moderate or severe (modified NYHA Class IIa, IIIb or IVc) congestive heart failure in dogs due to atrioventricular valvular insufficiency (AVVI) or dilated cardiomyopathy (DCM).
Safeheart is indicated for use with concurrent therapy for congestive heart failure (e.g., furosemide etc.) as appropriate on a case-by-case basis.
- A dog with modified New York Heart Association (NYHA) Class II heart failure has fatigue, shortness of breath, coughing, etc. apparent when ordinary exercise is exceeded.
- A dog with modified NYHA Class III heart failure is comfortable at rest, but exercise capacity is minimal.
- A dog with modified NYHA Class IV heart failure has no capacity for exercise and disabling clinical signs are present even at rest.
Howto take Safeheart?
Safeheart should be administered orally at a total daily dose of 0.23 mg/lb (0.5 mg/kg) body weight, using a suitable combination of whole or half tablet.
The total daily dose should be divided into 2 portions that are not necessarily equal, and the portions should be administered approximately 12 hours apart (i.e., morning and evening).
The tablets are scored and the calculated dosage should be provided to the nearest half-tablet increment.
Contradications
Safeheart should not be given in cases of hypertrophic cardiomyopathy, aortic stenosis, or any other clinical condition where an augmentation of cardiac output is inappropriate for functional or anatomical reasons.
Warnings
Only for use in dogs with clinical evidence of heart failure.
At 3 and 5 times the recommended dosage, administered over a 6-month period of time, pimobendan caused an exaggerated hemodynamic response in the normal dog heart, which was associated with cardiac pathology.
Human warnings
Not for use in humans. Keep this medication out of reach of children. Consult a physician in case of accidental ingestion by humans.
Precautions
The safety of Safeheart has not been established in dogs with asymptomatic heart disease or in heart failure caused by etiologies other than AVVI or DCM. The safe use of Safeheart has not been evaluated in dogs younger than 6 months of age, dogs with congenital heart defects, dogs with diabetes mellitus or other serious metabolic diseases, dogs used for breeding, or pregnant or lactating bitches.
Interactions
Safeheart may be used safely in dogs concurrently receiving furosemide, digoxin, enalapril, atenolol, spironolactone, nitroglycerin, hydralazine, diltiazem, antiparasitic products (including heartworm prevention), antibiotics (metronidazole, cephalexin, amoxicillin-clavulanate, fluoroquinolones), topical ophthalmic and otic products, famotidine, theophylline, levothyroxine sodium, diphenhydramine, hydrocodone, metoclopramide, butorphanol, and in dogs on sodium-restricted diets.
Side effects
Common side effects reported are poor appetite, lethargy, diarrhea, dyspnea, azotemia, weakness and ataxia, pleural effusion, syncope, cough, sudden death, ascites, and heart murmur.